Chemical Equations and Stoichiometry
Chemical Equations and Stoichiometry: Overview
This topic covers concepts such as Stoichiometry and Stoichiometric Calculations, Calculations Involving Mole-mole Relation, Calculations Involving Mole-weight Relation, Calculations Involving Mole-volume Relation, etc.
Important Questions on Chemical Equations and Stoichiometry
10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be:
The mass of carbon anode consumed (giving only carbon dioxide) in the production of 270 kg of aluminium metal from bauxite by the Hall process is (Atomic mass: ):
In the Haber process of dihydrogen and of dinitrogen were taken for reaction which yielded only of the expected product. What will be the composition of gaseous mixture under the aforesaid condition in the end?
In Haber's process, of dihydrogen and of dinitrogen were taken for the reaction, which yielded only of the expected product. What will be the composition of the gaseous mixture under the aforesaid condition in the end?
Assuming full decomposition, the volume of released at STP on heating (Atomic mass, ) will be:
Liquid benzene burns in oxygen according to the equation
How many litres of at STP are needed to complete the combustion of of liquid benzene?
Liquid benzene burns in oxygen according to the equation,
How many litres of at are needed to complete the combustion of of liquid benzene? (Molecular weight of )
One mole of magnesium nitride or reaction with an excess of water gives
For the reaction the percentage yield of product at different pressures is shown in the figure. Then. which among the following is true?
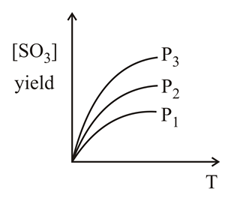
Calculate the amount of hydrogen gas required in order to produce of ammonia by the reaction of and gases.
Gastric juice contains about of per liter. If a person produces about liters of gastric juice per day, find the number of antacid tablets required to neutralize all the produced. Consider each antacid tablet contains of Given atomic masses:
The amount of arsenic pentasulphide that can be obtained when arsenic acid is treated with excess in the presence of conc. (assuming conversion) is
A gaseous hydrocarbon gives upon combustion of water and . What is the empirical formula of the hydrocarbon?
of an alkane requires of oxygen for its complete combustion. If all volumes are measured at constant temperature and pressure, the alkane is
When litres of is mixed with litres of each at STP, the moles of formed is equal to
If of oxygen combine with to form , the mass of in (Atomic mass of ) used in the reaction is
What weights of and will be produced by the combustion of of in of oxygen leaving no and .
In an experiment, pure carbon monoxide was passed over red-hot copper oxide. so produced, weighed and the weight of copper oxide was reduced by . Calculate the atomic weight of carbon. [ Take molar mass of carbon dioxide = ]
An alloy of aluminium and copper was treated with aqueous . The aluminium dissolved according to the reaction , but the copper remained as a pure metal. A sample of the alloy gave of measured at and pressure. What is the weight percentage of in the alloy?
Give the final answer after rounding off to two significant figures.
When a mixture of and is repeatedly digested with sulphuric acid, all the halogens are expelled and is formed quantitatively. With a particular mixture, it was found that the weight of obtained was precisely the same as the weight of mixture taken. Calculate the ratio of the weights of and in the mixture.
(Note: Report your answer after multiplying with 100 and rounding up to the nearest integer value.)
